In the pipeline industry, there are different types of corrosion that may occur, under certain conditions. Some of which include galvanic corrosion, alkaline acidic corrosion, and stress-corrosion cracking. There are multiple ways to reduce the risk of corrosion.
By Gobind N. Khiani, Fellow – Piping/Pipelines – Consultant
The Basic Types Of Corrosion Under Insulation
By understanding the types of corrosion that can occur under insulation (CUI), the proper materials and construction can be employed to prevent them. Intruding water is the key problem in CUI. Special care must be taken during the design stage, so as not to promote corrosion by permitting water to enter a system, either directly or in-directly. However, moisture may be external, or may be present in insulation.
Corrosion may attack the jacketing, the insulation hardware, or the underlying piping and equipment. Depending on other factors such as chloride, galvanic, acidic, or alkaline, corrosion may also occur.
Galvanic corrosion generally results from wet insulation, with an electrolyte or salt present that allows a current flow between dissimilar metals. Alkaline or acidic corrosion results when an alkali, or acid, and moisture, are present in certain fibrous or granular insulations. Chloride corrosion can be caused by the combination of insulation containing leachable chlorides, with the 300 series austenitic-stainless-steel surfaces. Stress corrosion cracking of insulating jackets is often the result of airborne salts in coastal regions.
Water entering the insulation and diffusing inward will eventually reach a region that is dried out, either at the hot pipe or equipment wall. Next to this dried out region is a zone in which the pores of the insulation are filled with a saturated salt solution, and this includes any chlorides present.
When a shutdown or process change occurs and the metal-wall temperature falls, the zone of saturated salt solution moves into the metal wall. Upon reheating, the wall will temporarily be in contact with the saturated solution (e.g., chlorides), and stress-corrosion cracking may begin.

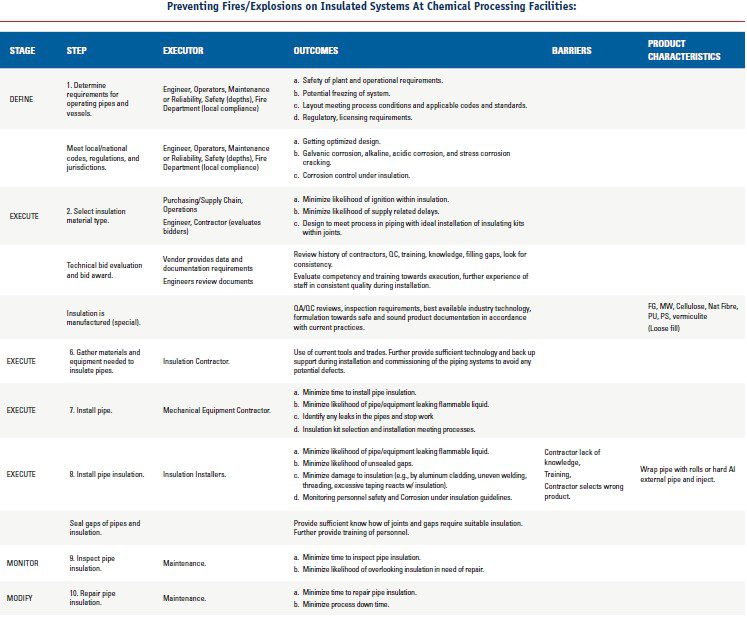
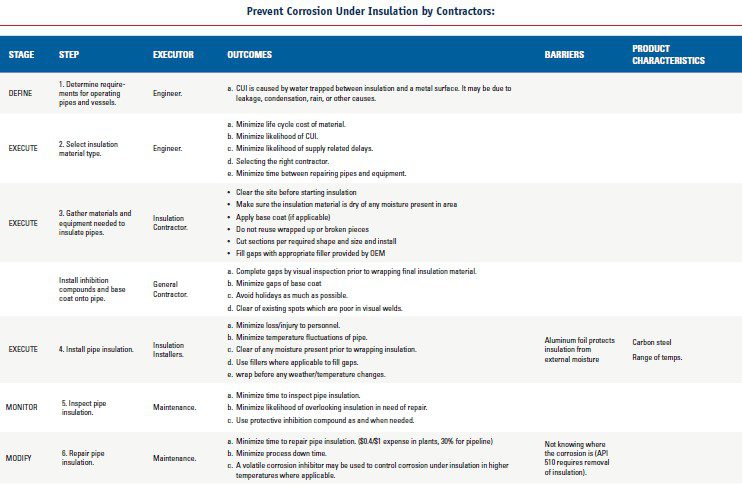
Preventing Corrosion
A major factor in preventing CUI is keeping liquid from intruding into the insulation. Water decreases the effectiveness of the insulation and leads to corrosion of the pipe or equipment. Poor conditions caused by wet insulation can be aggravated by weathering, vibration or improper use.
There are five factors in preventing CUI:
(1) Insulation selection.
(2) Equipment design.
(3) Protective paints and coatings.
(4) Weather barriers.
(5) Maintenance practices.
For example, if an area is subject to spills or high humidity, special consideration must be given to selecting the insulation. Some insulations leave the system less sensitive to defects in weatherproofing or paint films, because the insulations are non-absorbent and chemically nonreactive.
The Following Should Be Considered:
1. The cost of repairing the insulation if corrosion is detected.
2. The cost of the protective paint.
3. Use of non-absorbent insulation.
The proper design of insulation for pressure vessels, tanks and piping includes consideration of the support and connection of the material. The weather/vapor jacket of the insulation provides the primary barrier to water. This covering is the only part of the system that can be inspected quickly and repaired economically. It must not only keep liquids out, but also allow for evaporation of any liquid that manages to get into the insulation system. For weatherproofing, a rating of two perms, measured according to ASTM Standard E 398, is acceptable.
Additionally, it should be durable, offer flame-spread resistance, and be economical. The material must be maintained periodically (usually, two to five years) to remain effective.
Further, routine maintenance is needed to catch defects due to deterioration or misuse. If the system is opened in any way for maintenance or inspection, it should be closed promptly after work is completed. One plant reported that openings made in a metal vessel’s insulation for acoustic emission tests were never closed, and severe corrosion occurred.
Extensive use of a non-breathing metallic jacket is believed to contribute greatly to corrosion of warm equipment. Without a permeable jacket, water is trapped. Water in the insulation reaches a point where it is vaporized, and vapor then travels to the jacket and condenses; the cycle repeats itself.
REFERENCES
1. The National Board of Boiler and Pressure Vessel Inspectors.
2. Job Maps by James Rinne
3. H. Ahluwalia, “The Hidden Enemy: Corrosion Under Insulation.” Material Selection Resources Inc. presentation on 14 Nov. 2014, https://rbi1.gatech.edu/2014-corrosionsymposium-presentations.
4. S.A. Anderson, “Out of Sight, Out of Mind?” Hydrocarbon Engineering. August 2010, http://www.intertek.com/articles/2010-08-corrosion-under-insulation/.
5. R. Sampaio, A.L.M.V. Leite. “More Lesson Re-Learned from Corrosion Under Insulation,” 13 Mar. 2018. http://www. penderlo.com/ doc/Dow_CUI.pdf.
6. B. Bavarian, “Protection Effectiveness of Vapor Corrosion Inhibitor VpCI 619 for Corrosion Under Insulation at Elevated Temperatures.” California State University, Northridge, and Cortec Corp., Feb. 2018, https://www.cortecvci. com/Publications/Papers/CUI-report-on-VCI-619.pdf



