The word chlorine is often associated with the swimming pool chemical or bleach used as a household cleaning supply. However, chlorine (Cl), is an essential element that has multiple purposes and uses. In drinking water, chlorine is used to eliminate harmful bacteria and viruses such as cholera and typhoid. Nearly all modern drinking water systems use chlorine to prevent contamination. It is also a key ingredient in various medications that are used to treat diseases such as cancer, heart disease, malaria, and many other illnesses. A vast majority of herbicides and pesticides in the agriculture industry use chlorine. However, PVC resin and chlorinated solvent manufacturers are by far the largest users of chlorine. It is therefore important to understand the necessary specifications for the applications that are used to house and convey Cl.
By Alton Jamison, Sales Engineer – Teadit
Uses of Chlorine
Chemical plants use chlorine in the manufacturing of chemicals such as pesticides, plastics, and refrigerants. It is also a key ingredient in bleach, deodorizers, and disinfectants. Paper mills are large users of chlorine in the bleaching process of pulp, turning naturally brown craft paper into the white paper that we write and print on.
Chlorine History
Free elemental chlorine is almost nonexistent in nature, as almost all the Earth’s chlorine exists in the form of chemical compounds like sodium chloride (NaCl), also known as common or rock salt, which is the primary source of chlorine for industrial applications. Salt and other chlorine-containing compounds have had a multitude of uses dating back as far as 6,000 BC. However, the evolution of chlorine in the industrial environment and our everyday lives is a more recent story.

In 1774, Swedish chemist Carl Wilhelm Scheele produced free chlorine gas by reacting powdered black oxide of manganese with hydrochloric acid. Scheele began an intensive study of the properties and effects of the mysterious greenish-yellowish gas, noting its bleaching effect and deadly effects on insects, but failed to establish it as an element. Though Scheele is credited with its discovery, it was not until 1810 that Sir Humphry Davy first properly identified it as an element and named it chlorine, from the Greek ‘chloros’ meaning ‘green-yellow.’
In 1789, Count Claude-Louis Berthollet, a French textile producer, used the principles of the bleaching action of chlorine and bubbled it through a potash solution producing liquid chlorine bleach. In the 1830s, Sir Humphry Davy’s lab assistant Michael Faraday developed research on the electrolytic generation of chlorine and its liquefaction. Then in 1851, Charles Watt received a patent for an electrolytic chlorine production cell.
In the last two decades of the 1800s, progress was made in refining chlorine in multiple countries. By the 1900s the preferred method for chlorine production involved using mercury and diaphragm electrolytic cells, allowing the chlorine to be shipped as a liquid solution. In 1913, the first permanent chlorine water purification system was installed in Philadelphia. In 1924 a technical trade organization, The Chlorine Institute, was founded to support the safe operation of the chlor-alkali industry. By the 1930s, the chemical industry saw expanded use of chlorine in various applications. Today, one sees chlorine used extensively worldwide in the production of numerous products from pesticides and medications to bleaches and cleaners.
Chlorine Characteristics & Properties
In its physical state, chlorine is a gas at room temperature and becomes a liquid at -29°F. Elemental chlorine is often confused with liquid bleach because the two have become so synonymous in everyday vernacular. However, bleach is not the liquid form of chlorine, rather it is a dilute solution of the chlorine-containing compound sodium hypochlorite. Another common misnomer is that ‘chlorine tablets’ are the solid form of chlorine. They are, in fact, made from another chlorine-containing compound called calcium hypochlorite.
It is important to not confuse chlorine with the many products that utilize chlorine in their production. Gaseous chlorine is poisonous and classified as a pulmonary irritant, with the capability of causing acute damage to the upper and lower respiratory tract. The US Department of Transportation classifies chlorine as a class 2.3 inhalation hazard and bleach as a class 8 corrosive. Natural chlorine is non-flammable in its gaseous and liquid states; however, chlorine gas is a strong oxidizer and therefore can react with flammable materials.

The terms ‘wet’ and ‘dry’ chlorine refer to the moisture content, not the physical state. Dry chlorine is gas or liquid chlorine with less than 150 ppm of water. Wet chlorine, on the other hand, refers to gas or liquid chlorine-containing more than 150 ppm of water. Dry chlorine is strongly corrosive and reactive when moisture is present. Wet chlorine is corrosive to ferrous alloys and dry chlorine exhibits a strongly exothermic reaction with titanium. Prevention of moisture intrusion into ferrous dry chlorine equipment and piping systems is critical to the mechanical integrity of any chlorine plant.
Liquid chlorine properties:
- Clear amber in color;
- ~1.5x denser than water;
- Changes to a liquid below -29°F at atmospheric pressure;
- Freezes below -150°F at atmospheric pressure;
- High coefficient of volumetric expansion when changing from liquid to gas (460x).
Chlorine gas properties:
- Greenish-yellowish color at high concentrations;
- Yellow color at low concentrations;
- 2.5x denser than air;
- Can be very corrosive with water (do not spray water on a leak);
- Invisible below 25ppm.
Image 2 shows a controlled release of chlorine gas. Once released, the gas’s greater density causes it to remain close to the ground.
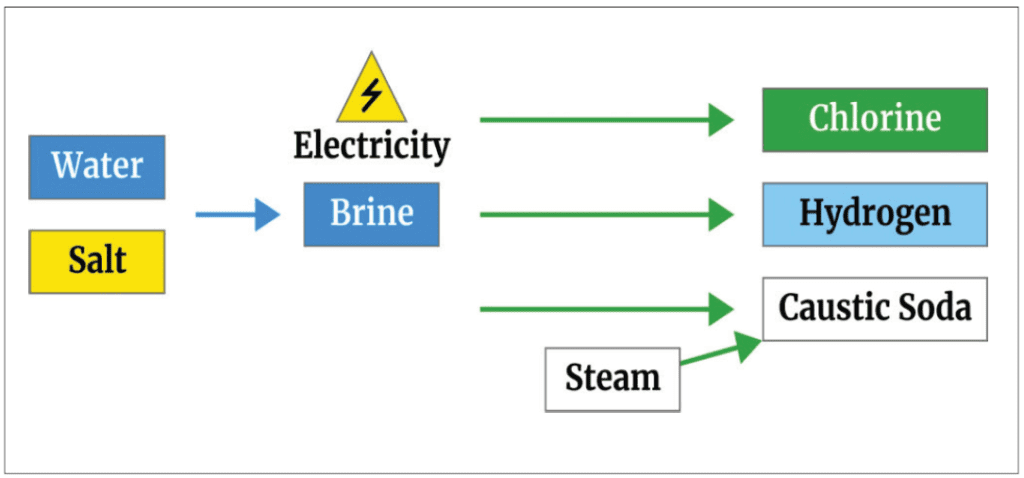
Chlorine Production
Chlorine is primarily manufactured by a diaphragm, membrane, or mercury cell process. Salt is electrolyzed by an electric current that converts chloride to elemental chlorine. Chlorine is normally shipped as liquefied compressed gas.

Chlorine Safety & Beyond
In September 2014, a chlorine packager had an accidental release at one of their facilities. A vapor cloud was discovered by several employees and the site went into emergency protocols. The chlorine cloud rose for three to five minutes and then fell to the ground. An investigation discovered that a gauge used on the equipment was not suitable for the service. This gauge had a tantalum diaphragm and titanium housing and looked identical to the stainless-steel gauges traditionally used for dry chlorine service. It was incorrectly pulled from the storeroom and utilized in dry chlorine service.
Unfortunately, as previously mentioned, chlorine reacts exothermically with titanium. This created a metal fi re which was believed to be the probable cause of the release. A corrective action was put in place to properly organize and separate the inventory to avoid gauges being placed into the incorrect services. This is just one example of the possible dangers related to working with chlorine.
Since its formation, The Chlorine Institute (CI) has been working to advance the safe, secure, environmentally compatible, and sustainable production, distribution, and use of its mission chemicals, including chlorine, sodium and potassium hydroxides, sodium hypochlorite, the distribution of vinyl chloride monomer (VCM), and the distribution and use of hydrogen chloride. Institute members are comprised of companies in the industry that make, transport and/or support the chloralkali industry. Together they publish guidance documents that address various topics relevant to the industry. These ‘pamphlet’ topics include things like piping and storage requirements, personal protective equipment, handling practices, and more.
Pamphlet 95 discusses suitable gasket technologies for use in wet and/ or dry chlorine services. It highlights several key considerations for proper gasket selection. It identifies chlorine as a highly aggressive oxidizer that reacts with numerous metals and organic compounds, and as such, highlights chemical compatibility as a key criterion for consideration. Additionally, it recommends proper installation practices to ensure sufficient loading throughout the service life of the gasket. Lastly, it specifies service condition criteria and provides listings of gasket materials that are suitable for installation within them.

When working with customers who are looking to find a gasket material that works in both wet and dry chlorine, TEADIT typically recommends Tealon® 1580 as a preferred choice. This material is Pamphlet 95 listed and is part of a family of materials commonly referred to as restructured PTFE. Restructured PTFE materials are designed to overcome the creep relaxation often associated with traditional skived PTFE sheets. They are produced with virgin PTFE resin combined with a filler material (in the case of TEADIT Tealon® 1580, barium sulfate) and then undergo a process that creates a uniform density within the sheet material and a high level of fibrillation throughout. Restructured PTFE materials manufactured utilizing this process are well-suited to handle the rigors associated with a wide range of chlorine and/ or chlorine-containing services as is evidenced by their inclusion in Pamphlet 95.
Final Thoughts
Chlorine is a critical element that plays an important role in the manufacturing and make-up of many products used in day-to-day life. When it comes to properly sealing industrial chlorine services, it is important to consider all of the information: temperature, pressure, assembly procedures, etc. It is critical that only sealing materials that can handle chlorine (wet and/or dry) at various states be chosen and that recommended best installation practices be followed. The pamphlets produced by The Chlorine Institute provide excellent guidance on the sealing, handling, and transportation of chlorine and are an invaluable resource to those working in the chlor-alkali industry, as the many uses of chlorine continue to expand and evolve.



