Petrochemicals are chemicals derived from petroleum and natural gas; and they are used in a variety of products, such as plastics, synthetic fibers, solvents, fertilizers, pharmaceuticals, explosives, and adhesives. In this article, we are going to learn more about these products are made – specially ethylene, one of the most important petrochemicals – and some of the valves commonly required for the process.
By Davi Sampaio Correia – Technical Consultant
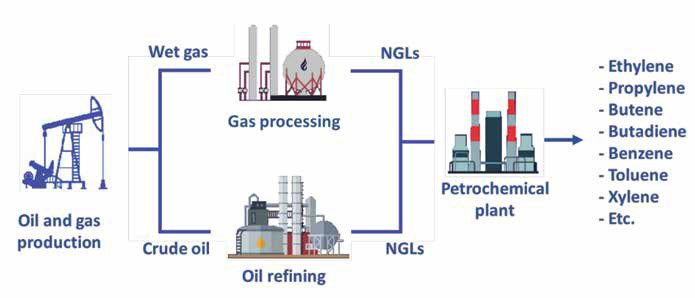
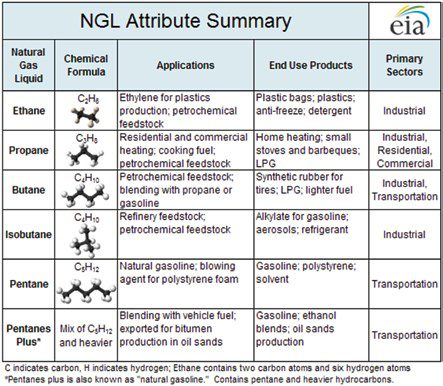
The term ‘Natural Gas Liquids’ (NGLs) typically refers to a group of hydrocarbons that includes ethane, propane, butanes, and natural ‘gasoline’ (see Figure 2). They are removed (condensed) as a liquid from a hydrocarbon stream that is typically in a vapor phase (for example, natural gas). NGLs remain in the liquid state for purposes of storage, transportation, and consumption.
In the United States, NGLs are mostly associated with natural gas production. Natural gas that is rich in NGLs and condensates is referred to as wet gas, while gas that is primarily methane, with little to no liquids in it when extracted, is referred to as dry gas. [2]
Over the last 20 years, advances in drilling and recovery technology – specifically, horizontal drilling and hydraulic fracturing – have transformed the energy market. The United States is now the largest producer of natural gas in the world, thanks to the exploration of its vast shale formations. [3] According to the EIA, U.S. natural gas production increased from 19 trillion cubic feet in 2005 to over 36 trillion cubic feet in 2019.
Recovering NGLs takes several steps. After it leaves the well, water and oil are removed from the natural gas, either at the producing site or at a nearby plant. Then, the gas is normally transported to a processing plant where it is further purified, that is, NGLs are removed, as well as other non-NGLs substances, such as sulfur, helium and carbon dioxide.
In chemistry, a feedstock is a raw material (input) fed into a process for conversion into something different (output). So, NGLs are feedstock for the production of petrochemicals. Six petrochemicals can be considered the fundamental building blocks of plastics: ethylene, propylene and butylenes, along with benzene, toluene and xylenes. NGLs are ‘the key ingredient in almost everything in our lives including building materials, bicycles, plastic bottles, shopping bags, car parts, heating fuels, carpeting, synthetic fabrics, medications, skis, snowboards, hiking boots, backpacks and even baby diapers.’ [2]
Of the six petrochemicals mentioned earlier, one is considered the ‘king’ of them all – ethylene. This is because ‘more commercial chemicals are produced from ethylene than from any other intermediate. This unique position of ethylene among other hydrocarbon intermediates is due to some favorable properties inherent in the ethylene molecule as well as to technical and economic factors. These could be summarized in the following:
• Simple structure with high reactivity.
• Relatively inexpensive compound.
• Easily produced from any hydrocarbon source through steam cracking and in high yields.
Ethylene Production and Valves
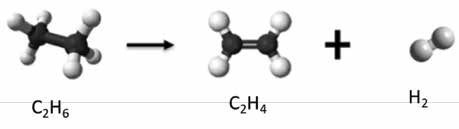
The conversion of complex, long-chain, hydrocarbon molecules to simpler, light, molecules is known as cracking, which is done by breaking the carbon-carbon bonds in the original molecule. Figure 3 shows this concept for the conversion of ethane to ethylene, yielding hydrogen as well.
Around the world, ethylene production is normally based on thermal cracking of either naphtha (liquid feed, composed mostly of pentanes and hexanes) or ethane (gas feed). Whatever the feed, thermal cracking usually involves feedstock and steam being injected into a furnace and staying there for a specific time; this process is called steam cracking (or pyrolysis). ‘Other processes for the production of ethylene are recovery from FCC off-gas, FCC cracking, catalytic pyrolysis, and coal-to-olefins and methanol-to-olefins conversions.’ [6]
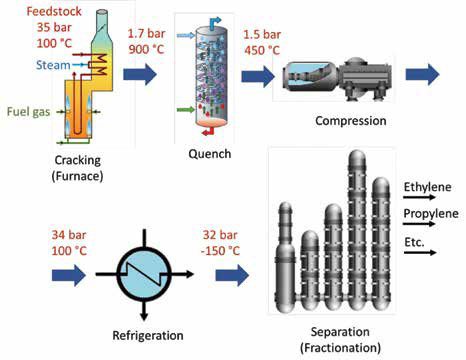
Figure 4 presents a simplified block diagram for ethylene production. Pressure and temperature during several of the steps are shown for a typical steam cracking process.
The process can be summarized as follows. Feedstock (liquid or gas) and steam are introduced into a furnace, where high temperatures – as high as 900°C – are used to crack hydrocarbons. Upon leaving the furnace, the stream is abruptly cooled down, to preserve the current composition and avoid undesirable side reactions. A compression and condensation section increases pressure and helps in removing heavier components from the stream. After the last compression stage, the cracked gas is chilled to cryogenic temperatures and sent to the separation towers. These are a set of distillation columns where ‘each of the subsequent columns receives the bottom feed of the previous column, starting with the De-methanizer or Deethanizer column and ending with the De-butanizer column.’ [6]
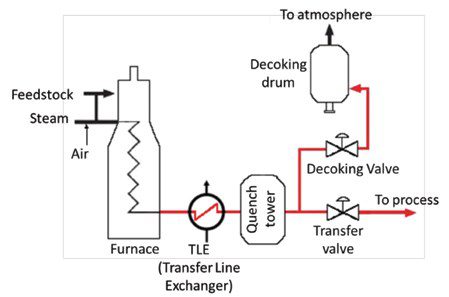
Of all the valves required, two face the direst conditions: the transfer line valve and the decoke valve. To better understand the challenges they face, let’s first talk about coke. As we have seen, ethylene is produced by cracking long-chain hydrocarbons into smaller, short-chained molecules. A by-product of cracking is coke – a carbon-rich solid material that is continuously deposited on the heat transfer surfaces of the furnace and it is also carried away in the stream leaving the furnace. Steam acts like a coking inhibitor, since it reacts with coke to form carbon dioxide (CO2), carbon monoxide (CO), and hydrogen (H2).
Steam injection is not enough to completely suppress coke formation and decoking cycles are required from time to time. ‘This is typically done by burning out the coke with a mixture of steam and air. Time intervals for decoking will depend upon several factors including, but not limited to the type of furnace, how the process is operated, feedstock type, and the types of coils utilized.’ [5]
The decoking operation rely on two valves: the transfer line valve and the decoke valve (see Figure 5), whose positions must be reversed. During normal operation, the transfer line valve remains open, but for decoking it is closed. In a similar but reverse manner, the decoking valve is normally closed and now must be open. The change in the valves’ position must be carefully controlled, for ethylene is a volatile product and ‘has a low flash point, therefore imposing a fire and explosion risk if it is vented to the atmosphere.’ [7]
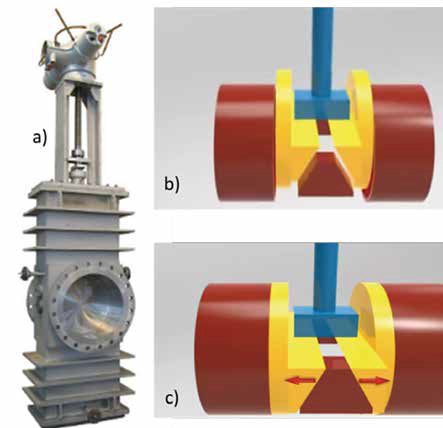
In short, these two valves must operate under high temperature (with occasional thermal cycling), provide good sealing, be able to withstand hard particles buildup/ erosion, and lastly, to resist possible corrosion from a low pH environment. All this while providing excellent reliability in synchronized operation- remember, the opening of one and the closing of the other must happen flawlessly due to the fire hazard previously mentioned.
Conclusion
With temperatures ranging from the cryogenic to inducing ‘cherry red hot’ hues in steels, steam cracking is a very challenging process for valves and associated equipment. Transfer line valves and decoking valves bear the brunt of it due to a unique combination of demanding environment (low pH, high temperature, erosion and coke build-up) and the need of flawless operation. For such cases, through-conduit gate valves are a common choice.
REFERENCES
8. https://guichon-valves.com/transfer-line-anddecoke-valve
About the Author


