The global energy market is changing rapidly due to the energy transition. Satisfying the growing energy demand while reducing the risks of global warming and social costs from climate change is a big challenge in the energy economy. Carbon Dioxide, Methane, Nitrous Oxide, and Fluorinated Gases cause the earth’s global temperature to rise. As each gas can remain in the atmosphere for different periods of time and for each greenhouse gas, a Global Warming Potential (GWP) measurement was developed to compare the impacts of different gases on global warming.
By Naomi Jabbari, Heat Transfer Engineer – S&B Engineers and Constructors
The GWP is a great indicator of how much energy the emissions of one ton of a gas will absorb over a specific period. A comparison of the emissions of one ton of carbon dioxide (CO2) is below:
- CO2 is the reference gas for GWP and has a GWP of 1 regardless of the length of time it is used. CO2 emissions cause increases in atmospheric concentrations of CO2 that remain for thousands of years.
- Methane (CH4) has a GWP of 27-30 over 100 years. CH4 absorbs much more energy than CO2, however, it lasts much less time than CO2; the effect of the shorter lifetime and higher energy absorption has been considered in GWP.
- Nitrous Oxide (N2O) has a GWP of 273 over 100 years.
- Chlorofluorocarbons (CFCs) with different GWPs include hydrofluorocarbons (HFCs), hydrochlorofluorocarbons (HCFCs), perfluorocarbons (PFCs), and sulfur hexafluoride (SF6) are called high-GWP gases.
A Closer Look at Global Warming Potential Indicators
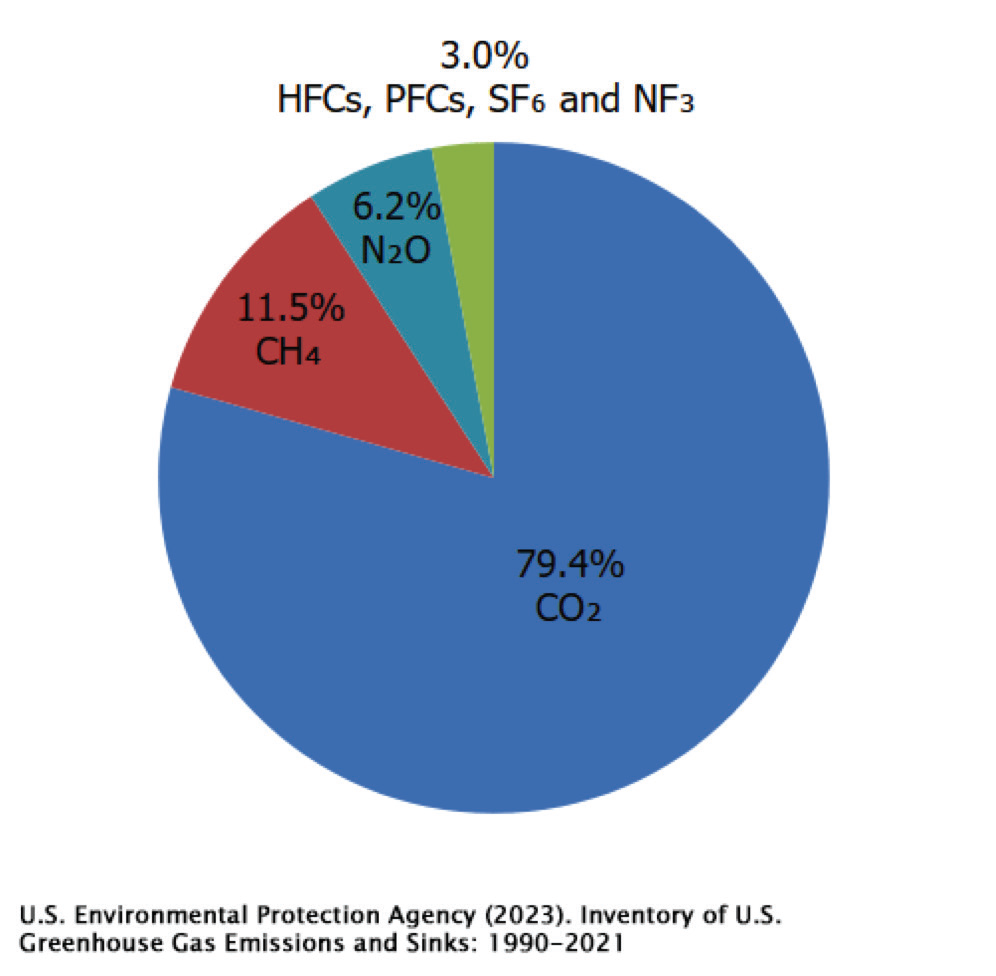
GWP provides an indication of the stronger impact some gases have over others, in regard to their effect on global warming. It is clear that Carbon Dioxide is the main greenhouse gas and ac- counts for roughly 79% of total greenhouse gas emissions which trap infrared and increase the earth’s temperature.
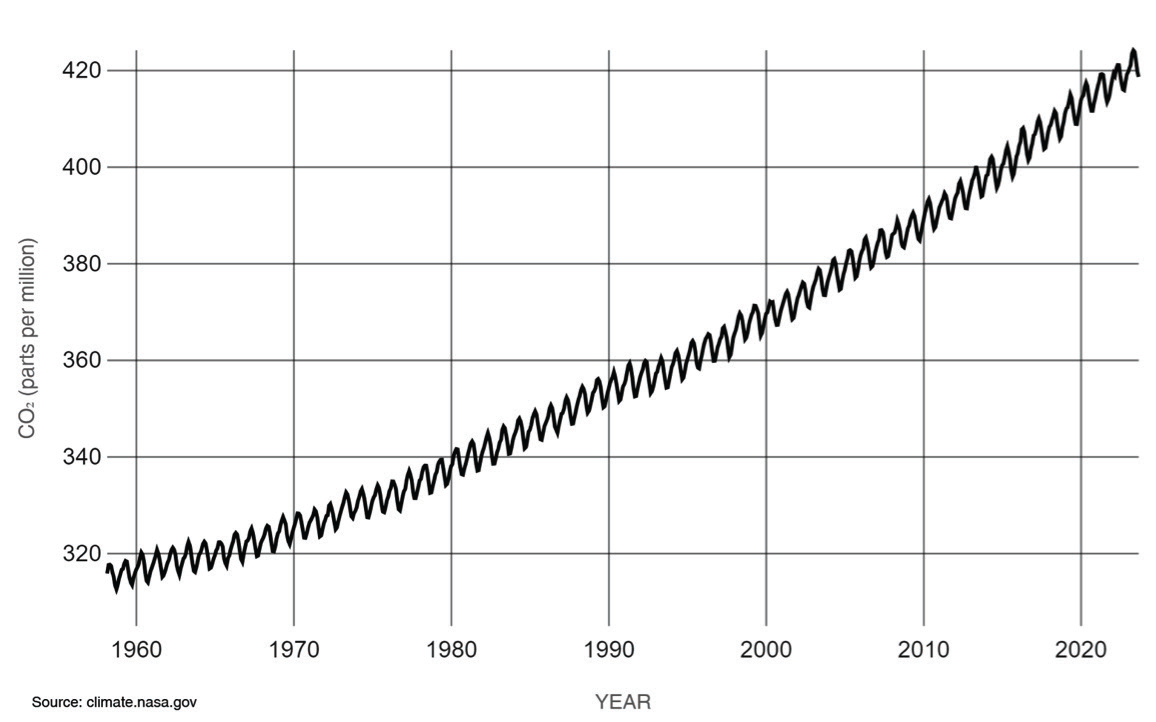
CO2 comes from the burning of fossil fuels and natural processes like volcanic eruptions. The natural carbon cycle normally keeps CO2 levels in balance, but human activities, mostly the burning of fossil fuels, produce more CO2 than nature can absorb. Human activities have raised atmospheric CO2 by 50%; the amount of CO2 is now 150% of the value it was in the year 1750, per NASA’s reports. Figure 2 illustrates the significant rise in atmospheric CO2 concentrations over time.
Fossil resources themselves are not the reason for global warming. Rather it is the CO2 from the combustion of fossil fuels that causes issues with climate change. An energy transition to reach net zero carbon dioxide emission by 2050 is needed to address this challenge and provide opportunity in the energy economy by utilization or sequestration of CO2 instead of releasing it to the atmosphere. The new energy market for Carbon Capture Utilization and Storage opens the doors for research and development of new technologies and allows for the optimization of existing energy infrastructure; it will be a revolution in the energy market at the global level.
Innovation efforts are developing to mitigate CO2 emissions in heavy industries. Low-carbon production of steel, chemicals, and hydrogen, as well as lower-carbon binding materials in cement production and employing inert anodes for primary aluminum production, are innovative ways to reduce CO2 emissions for heavy industry.
What is Carbon Capture?
The carbon capture process is a complex gas separation process. It uses Chemical Absorption, Adsorption, or Membrane separation and can be done for any stationary CO2, including oil & gas, biofuel, hydrogen production from steam methane reforming process, and other methods. The process includes Feed Dehydration, CO2 capture, and CO2 Compression. All of the processes need mass and heat transfer, and it is obvious that flow control systems play a significant role in the capture process.
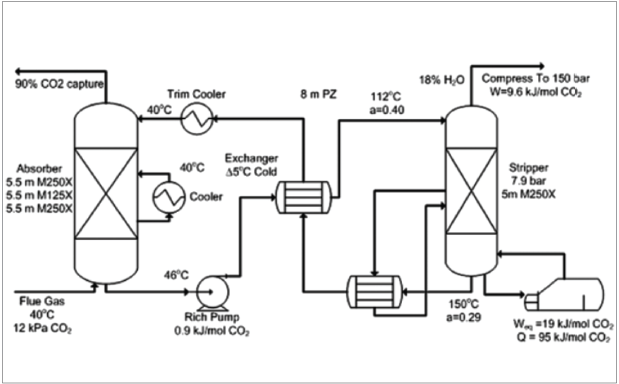
Figure 3 is an example of the CO2 absorption process: Gas absorption to remove CO2 from flue gas will happen in an absorption column and liquid stripping (desorption) to remove CO2 from amine solvent will be done in a stripper column. As this process is based on gas-to-liquid mass transfer, the design of heat transfer equipment and flow control systems including valves is important to optimize heat integration and minimize utility and investment costs.
In the chemical absorption process for post-combustion capture, which is the main commercialized capture method, CO2 is separated from the exhaust gases using a chemical sorbent at the end of the process. Due to high sorbent costs, additional thermal energy is required to regenerate the saturated sorbent to make it reusable. Key challenges to having a sustainable capture process include reducing the additional energy cost of the capture unit by improving sorbents to minimize degradation and decrease the project costs. A CO2 compression process is required after capture and coordinating efforts should focus on developing CO2 transport infrastructure and the identification of storage sites needed if sequestration is the final purpose of the capture process.
Other CO2 Capture Methods
Another capture method is an adsorption process based on gas-to-solid mass transfer and accumulation of CO2 on the surface of a solid such as Zeolites/ Metal-Organic Frameworks (MOFs). Adsorption-Desorption Cycle includes main 4 steps: pressurization, adsorption, forward blowdown, and reverse evacuation of CO2 based on PSA (pressure swing adsorption), VSA (vacuum swing adsorption) and TSA (temperature swing adsorption) methods. The high cost of advanced capture materials, such as Zeolites and optimized processes technology, are the main challenges for this method. Membrane separation is also a capture method and is considered a multistage capture based on a diffusion process that causes particles to move from a high-concentration area to a low-concentration area.
The diversity of the Stationary CO2 recourses dictates different CO2 capture processes for each project since they have different feed composition with different CO2 purity and CO2 recovery. The boost in CCUS projects in recent years helps different capture methods to get more cost-effective compared to a few years ago when the technologies were very new in the market.
The CCUS projects are increasing globally and there are some main reasons for that. New policies and incentives such as the Inflation Reduction Act in the United States, the Net Zero Industry Act in the European Union, and the 14th Five-Year Plan already in place in China –significantly accelerate funding and investments on CCUS projects.
Growing recognition that CO2 capture, utilization or sequestration are necessary to meet net zero goals are combined with growing interest in producing low-carbon hydrogen and synthetic fuels such as sustainable aviation fuels in energy market help to scale up investments on new technologies related to capture processes.
Final Thoughts
In conclusion, Net Zero by 2050 is a feasible solution for the global warming problem, and the goal is to reduce greenhouse gas emissions, especially carbon dioxide from the energy sector with minimum social costs. The carbon capture market will continue to grow to support increasing global energy and non-energy demand.
New CO2 utilization markets are developing to support renewable and low-emission energy projects, especially biofuels, ammonia, and hydrogen pro- duction. This opportunity also provides new markets for existing flow control and heat transfer industries to support the energy transition. It is obvious a cleaner, resilient, and sustainable future energy system with net-zero emissions will require a wide range of new technologies in the carbon capture market which is still at an early stage of development in the very competitive net-zero market.
References:
- https://climate.nasa.gov/vital-signs/carbon-dioxide/#:~:text=Key%20Takeaway%3A,in%20less%20 than%20200%20years.
- https://www.climate.gov/news-features/understanding- climate/climate-change-atmospheric-carbon-dioxide
- https://www.iea.org/energy-system/decarbonisation- enablers/innovation
- https://heat-exchanger-world.com/wp-content/ uploads/sites/19/2022/04/Jabbari.pdf
- https://pubs.rsc.org/en/content/articlehtml/2013/ee/ c3ee42350f
- https://www.epa.gov/ghgemissions/overview- greenhouse-gases
- https://www.sciencedirect.com/science/article/pii/ S277282342200001X#:~:text=In%20numerous%20 processes%20studied%20for,compared%20to%20 conventional%20separation%20processes.
- https://www.ncbi.nlm.nih.gov/pmc/articles/ PMC9277071/#:~:text=Common%20adsorption%20 carbon%20capture%20processes.&text=Pressure%20 swing%20adsorption%20technology%20is,pressure%20 under%20constant%20temperature%20conditions.



